My career
the short history
This is this short history of what I did.
Other posts will tell what I learned.


I started my career working in a neurosurgery department in the 1970s, doing intraoperative monitoring for spine cases. The equipment in those days had to be “home made” from a signal averager, a paper tape reader for programs, and a pen-and-ink plotter for output, all bolted to a large moveable equipment rack. I got the idea that this could be made smaller, cheaper, and better using microprocessors which were very new at that time.
I happened to be at the right place at the right time and teamed up with a gifted and skilled electrical engineer, Gary Trapuzzano, while working at Interspec, Inc., and the Neurotrac product line was born.

The Neurotrac was the first microprocessor based instrument for performing EEG, processed EEG, and somatosensory evoked potentials. It was used clinically in surgery and critical care in hospitals around the world during the 1980s. It became the first commercial instrument to trend a processed EEG parameter, the Spectral Edge Frequency (SEF), due to an early friendship with Dr. Ira Rampil, the inventor of the SEF. Data collected using the Neurotrac found its way into numerous research publications and many textbooks. A training booklet I wrote became a widely used teaching tool in anesthesia training programs and for most EEG monitor manufacturers.

The original Neurotrac, the first microprocessor based instrument for performing EEG, processed EEG, and somatosensory evoked potentials
In 1990, I spun off my own company, Moberg Medical, from Interspec and started developing the Neurotrac II. The Neurotrac II, provided 8 channels of continuous EEG collection and storage, along with EEG processing to compute graphical trends. It was the first product to provide EEG and evoked potentials simultaneously and also collect data from a transcranial Doppler ultrasound (TCD) machine. A unique feature of the Neurotrac II came from a partnership with Hewlett-Packard. The system used the display and user interface of the most popular bedside monitor at the time, the HP CMS. Because of its similarity to a vital signs monitor, it was noted to be the EEG monitor that was the easiest to use by anesthesia and critical care personnel. The Neurotrac II was used primarily in surgery but increasingly in critical care by pioneers such as Dr. Tom Bleck and Dr. Paul Vespa. The company was sold in early 1998 to Telefactor.

The Neurotrac II, the first product to provide simultaneous EEG and evoked potentials and also collect data from a transcranial Doppler ultrasound (TCD) machine
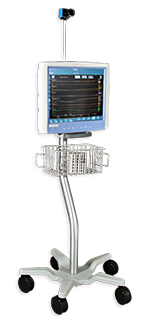
The Component Neuromonitoring System (CNS Monitor), the first device to be FDA cleared to provide EEG integrated with data collected from other monitors
In 1998, I started Moberg Research, to begin developing a new concept and basic platform for integrated neuromonitoring. The Component Neuromonitoring System (CNS Monitor), built upon two concepts learned from the Neurotrac II. First, users need to see the “big picture” of brain activity on one screen like they do with hemodynamic data on a vital signs monitor. Thus, the system was designed from the start to be a general purpose data collection and time-synchronization system. Second, the monitor needs to be easy to use by clinical personnel at the bedside. As a result, a “vital signs monitor” user interface was adopted and a new way of linking instructional content to the time of need, called InfoMap®, was developed.
The development of the CNS Monitor concept and system was supported by several SBIR grants from NIH, as were some specific applications of the system in neonatal monitoring. In the early development of the product, technology was licensed by Integra LifeSciences. They used it to produce the Mobius monitors, which collected and integrated data from two Integra products and a vital signs monitor. Development of the CNS Monitor continued as the company sought to achieve seamless data collection and integration of medical devices, the implementation of which proved more difficult than originally anticipated. The goal was reached and the CNS Monitor was cleared by the FDA in November of 2008, two months into a historic recession. The company continued research and development activities as well as work in diversified business units as the market recovered. The CNS Monitor emerged to yield a profitable and rapidly growing business.
The company rebranded itself as Moberg ICU Solutions to reflect its mission more clearly. The company moved to the forefront of integrated neuromonitoring and developed core technology for a new information architecture for patient management at the bedside. We received funding from NIH and the Department of Defense to further develop ideas. The product was sold into some major hospital accounts with some placing the product at every bed in a neuro ICU. In 2019, the company was acquired by ArchiMed, a large European private equity group via Micromed, one of its portfolio companies. And in 2023, Micromed was acquired by Natus, the worldwide leader in neurophysiology.
In 2020, in the middle of the pandemic, I started Moberg Analytics to focus on how to maximize the value of data from the injured brain once the CNS Monitor collects it. I joined the company full time in 2021 when my contract with Micromed ended. I am now working with an amazing group of colleagues building a cloud platform that will help clinicians and researchers learn more about brain injury and manage it more precisely.